With AbTheneum, we can confirm if these cell populations also secrete IgG. Using a microfluidic-based sorter, to sort the 3 populations of CD138+ cells from the immunized wild-type mice. All 3 populations show that a majority still express IgG on the surface. We further enriched the CD138-high population (in green) for CD138-high and the antigen, Human Serum Albumin (HSA) in a 4th population. The 4 populations and their CD138 and antigen expression are shown in Figure 3.
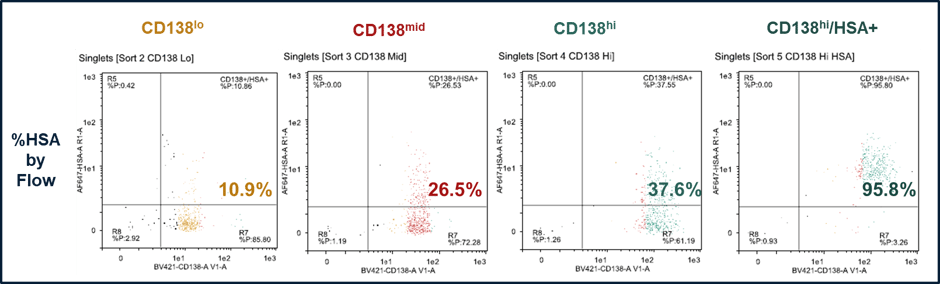
Figure 3. Four populations of sorted mouse lymph node cells with increasing CD138 expression, showing their % antigen positive (antigen is HSA). The CD138-hi/HSA+ population was sorted for high expression of CD138 and HSA.
The secretion rate of all 4 sorted populations was confirmed using AbTheneum's antibody capture & screen workflow (Figure 4). The results confirm that CD138-high has the highest hit rate (HSA-positive/all IgGs). We also confirmed that most CD138-hi cells also express IgG on the surface, and further enriching for antigen increases the hit rate.
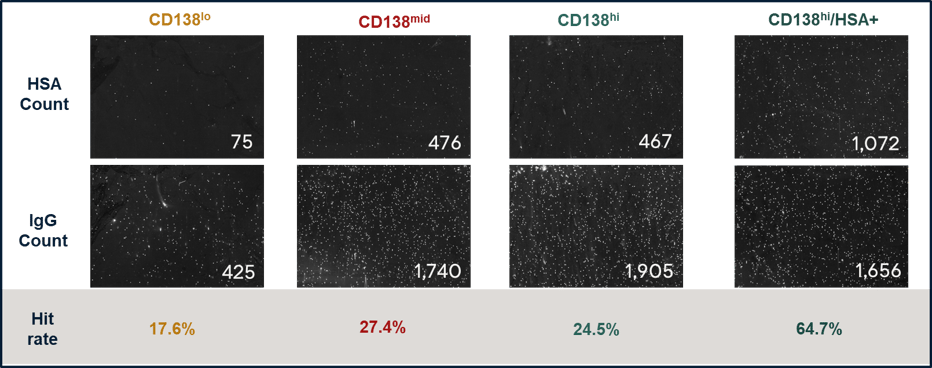
Figure 4. Four sorted cell populations captured antibodies with AbTheneum screening. A small cropped image of the antibody screening assay are shown with the total count of anti-HSA hits and total IgGs, detected by fluorescent labeled HSA and a fluorescent secondary antibody, respectively. The hit rate for each cell population is the ratio of HSA hits/all IgGs.
This study establishes a workflow we call FACS antigen+, where we can isolate antibody-secreting, antigen-specific cells from immunized animals. To test the workflow, we ran the FACS antigen+ sorting on several targets with head-to-head compared with traditional magnetic activated cell separation (MACS) for CD138-high expressing cells.
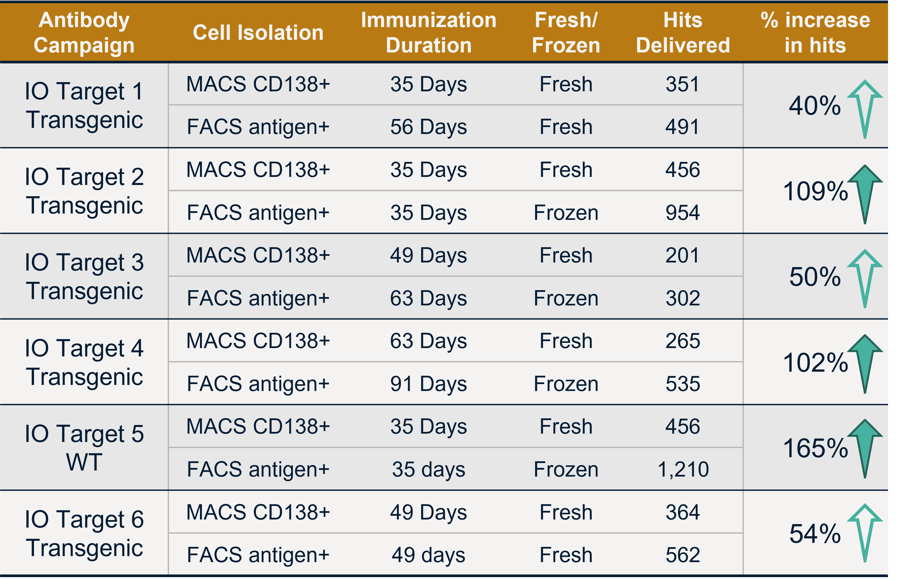
All trials using the FACS antigen+ cell isolation delivered more hits (antibody sequences of screened antigen-positive antibodies), even in less ideal samples like previously frozen or low titer samples. Three of the 6 trials delivered >100% hits compared to magnetic CD138+ cell isolation, highlighting the power of FACS antigen+ workflow with AbTheneum antibody discovery. Table 1 shows results from 6 head to head studies ran with IO targets and the increase in hit rate.
Table 1. Six diverse IO targets were tested in head-to-head comparisons of commercial CD138+ cell isolation kits vs. FACS antigen+ workflow. Hit rates are the number of antigen-specific antibody sequences delivered for each campaign.
Impact: What We Achieved
FACS antigen+ is able to increase the total hit count of campaigns, even in low abundance targets. The workflow is rapid and fully compatible with AbTheneum antibody discovery, requiring no changes to downstream processes. In comparative analyses of head-to-head campaigns, identical clones and related clonotypes were recovered from both FACS antigen+ and MACS CD138+ cell isolation pools, indicating that the FACS antigen+ workflow preserves antibody repertoire diversity in the final output.
Would you like to know more?








